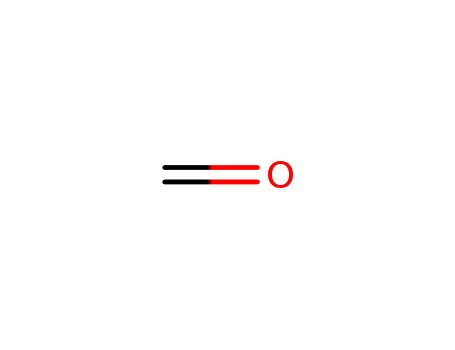
NewblueCHEM--High purity Paraformaldehyde factory price CAS NO.30525-89-4
- FOB Price: USD: 100.00-100.00 /Kilogram Get Latest Price
- Min.Order: 1 Gram
- Payment Terms: L/C,T/T
- Available Specifications:
1(1-100)Kilogram
- Product Details
Keywords
- High purity CAS 30525-89-4
- 30525-89-4
- NewblueCHEM--High purity Paraformaldehyde factory price in stock
Quick Details
- ProName: NewblueCHEM--High purity Paraformaldeh...
- CasNo: 30525-89-4
- Molecular Formula: C3H6O3X2
- Appearance: White solid
- Application: medical
- DeliveryTime: In Stock
- PackAge: 25KGS/Drum
- Port: China Main Port
- ProductionCapacity: 10 Metric Ton/Day
- Purity: 99%
- Storage: Room temperature
- Transportation: BY SEA
- LimitNum: 1 Gram
- Moisture Content: N/A
- Impurity: N/A
- Grade: Industrial Grade,Pharma Grade
- Assay: 99%
Superiority
Our main business covers the fields below:
1.Noble Metal Catalysts (Pt.Pd...)
2.Organic Phosphine Ligands (Tert-butyl-phosphine.Cyclohexyl-phosphine...)
3.OLED intermediates (Fluorene,Carbazole,Boric acid...)
4.Pharmaceutical intermediates
About us
1.More than 10 years chemical exporting experience
We have produced chemical more than fifteen years., 80% products are for export .
More than 10 years chemical exporting experience. Good and stabilized factory price.
2.Strict quality control system
3.Supply sample
Before order , we can send the sample for your testing . We ensure the quality is the same as bulk quantity .
Our advantage:
Q1:Can I get the free sample
A: Yes, we can supply the free sample, but the shipping cost be paid by our customers.
Q2: How to start orders or make payments
A: Proforma invoice will be sent first after confirmation of order, enclosed our bank information.
Payment by T/T, or Paypal or L/C.
Q3: How to confirm the Product Quality before placing orders
A:You can get free samples for some products,you only need to pay the shipping cost or arrange a courier to us and take the samples.
You can send us your product specifications and requests,we will manufacture the products according to your requests.
Q4:What’s your MOQ
A:Usually according you demand.
Q5: How about delivery leadtime
A:Delivery lead time: As soon as possible. (Chinese holiday not included)
Q6:Is there a discount
A:Different quantity has different discount.
Q7: How to contact us
A:You can chat with us by Trademanager,Email,WhatsAPP,Wechat.
You can choose your interested products and send inquiry to us.
You can dial our telephone directly, you will get our reply.
Details
Packing:

About us
1.More than 10 years chemical exporting experience
We have produced chemical more than fifteen years., 80% products are for export .
More than 10 years chemical exporting experience. Good and stabilized factory price.
2.Strict quality control system
3.Supply sample
Before order , we can send the sample for your testing . We ensure the quality is the same as bulk quantity .
Delivery:


Our advantage:
Q1:Can I get the free sample
A: Yes, we can supply the free sample, but the shipping cost be paid by our customers.
Q2: How to start orders or make payments
A: Proforma invoice will be sent first after confirmation of order, enclosed our bank information.
Payment by T/T, or Paypal or L/C.
Q3: How to confirm the Product Quality before placing orders
A:You can get free samples for some products,you only need to pay the shipping cost or arrange a courier to us and take the samples.
You can send us your product specifications and requests,we will manufacture the products according to your requests.
Q4:What’s your MOQ
A:Usually according you demand.
Q5: How about delivery leadtime
A:Delivery lead time: As soon as possible. (Chinese holiday not included)
Q6:Is there a discount
A:Different quantity has different discount.
Q7: How to contact us
A:You can chat with us by Trademanager,Email,WhatsAPP,Wechat.
You can choose your interested products and send inquiry to us.
You can dial our telephone directly, you will get our reply.
Our Factory






 Assessedsupplier
Assessedsupplier


